One does not simply destroy a nuclear weapon
It's the isotopes...
Posted by ekr on 05 Dec 2022
In a recent article the NYT reports that in the US when nuclear weapons are retired they aren't destroyed but just stored:
Typically, nuclear arms retired from the U.S. arsenal are not melted down, pulverized, crushed, buried or otherwise destroyed. Instead, they are painstakingly disassembled, and their parts, including their deadly plutonium cores, are kept in a maze of bunkers and warehouses across the United States. Any individual facility within this gargantuan complex can act as a kind of used-parts superstore from which new weapons can — and do — emerge.
...
“It’s important to keep these parts around,” said Franklin C. Miller, a nuclear expert who held federal posts for three decades before leaving government service in 2005. “If we had the manufacturing complex we once did, we wouldn’t have to rely on the old parts.” He added that other nuclear powers can and do make new atomic parts.
I'm not really surprised that the weapons aren't being destroyed because it's incredibly hard to do so in a meaningful fashion; it's not like guns where you just melt them down or something. However, seeing why requires an understanding the physics of the situation, so let's start there.
Thanks to Wikipedia, which was indispensible in gathering the background detail for all this. I also can't recommend enough Richard Rhodes's The Making of the Atomic Bomb, which provides a very clear account of the physics of nuclear weapons, as well as the history of the Manhattan Project.
Backgrounder: Atoms, Elements, and Isotopes #
This section is elementary but important material on the structure of matter. If you know what an "element" and an "isotope" is, you can skip this.
Essentially all ordinary matter—the stuff you are made of and encounter on a daily basis—is composed of atoms. An atom is composed of three basic subatomic (i.e., smaller than atoms) particles:
- Positively charged protons
- Negatively charged electrons
- Non-charged neutrons
At a super-simplified level, an atom is like a miniature solar system, with a nucleus at the center, consisting of protons and neutrons, and the electrons orbiting around it.[1] Atoms have the same number of electrons and protons, which renders them neutrally charged. An atom can also gain or lose an electron to become an ion, which is something we'll need to know later.
The chemical properties of an atom are dictated by the number of electrons, and because the number of electrons is the same as the number of protons in the nucleus, the number of protons also dictates those properties. Every atom with a given number of protons in the nucleus (the atomic number) thus has the same chemical properties (the technical term here is element). Each element has a name and a one or two letter symbol. For instance, hydrogen's symbol is "H", oxygen's is "O", etc. There are 100 or so elements, but of course many more chemicals because you can combine elements in a lot of different ways.
Finally, this brings us to neutrons. It's possible to have different numbers of neutrons in the nucleus of an atom, even with the same number of protons. For instance, you can have three different flavors of hydrogen atoms:
| Name | Number of Neutrons |
|---|---|
| Hydrogen | 0 |
| Deuterium | 1 |
| Tritium | 2 |
Because the neutrons have no impact on the charge of the nucleus, they also have no influence on the number of electrons, which means that all three types of hydrogen have basically the same chemical properties; they just have different masses. The term for different flavors of the same element is isotope, as in "deuterium and tritium are two different isotopes of hydrogen". It's standard to refer to isotopes by the total combined number of neutrons and protons in the nucleus, so, for instance, deuterium is H-2 (H for hydrogen).[2] Many elements exist in multiple isotopes in nature, though in many cases one isotope is common and the others are rare.
Brief Overview of the Physics of Nuclear Weapons #
I said above that chemical reactions don't create or destroy atoms, but it's possible to have nuclear reactions which do exactly that. There are several such processes.
Atomic Decay #
Many atomic isotopes are unstable, which means that they will spontaneously decay into other isotopes by emitting some other particle. For instance, the element uranium-238 decays by emitting an alpha particle (another name for a helium nucleus, containing two protons and two neutrons), reducing the atomic number by two (the two protons) and the atomic weight by four (the two protons plus the two neutrons) and giving you the element thorium-234. Thorium is itself unstable and decays by emitting a beta particle (another name for an electron, see radiation) to give you protactinium-234m.[3]
Different isotopes decay at different rates. The standard way to define this in terms of what's called a "half-life", which is to say the amount of time it takes half of the atoms in a given sample of an isotope to decay (alternatively, the time after which there is a 50% chance that a single atom has decayed). Shorter half-lives mean that an isotope is more radioactive (because there are more decays per second); longer half-lives mean that they are more stable. It's possible to have isotopes with very long half lives, on the order of thousands of years. Note that atomic decay is effectively a memory-less process, which is to say that if you start from X units of an unstable isotope, it takes the same amount of time to get from X to 1/2 X as it does to get from 1/2 X to 1/4 X.
In addition to releasing particles, this process releases energy, in various forms, including:
-
Kinetic energy from the new atom and the emitted particle moving faster than they were before. These particles then interact with the surrounding material, producing heat.
-
Radiation in the form of x-rays, neutrons, etc.
This means that radioactive isotopes tend to be warm or even hot. In fact, it's possible to exploit this effect to power devices for long periods of time in what's called a radioisotope thermal generator (RTG). RTGs are a common way to power spacecraft, for the obvious reason that you can't easily get out there and change the batteries.
One thing to notice here is that this is a one-way process, with unstable elements decaying to produce other lighter elements and energy. Eventually, the process terminates when some relatively stable isotope is produced, at which point you have a stable system and a bunch of heat: see also the second law of thermodynamics. It's also possible to go from lighter to heavier products, as we'll see below in the discussion of fusion.
Radiation #
You'll often hear that various isotopes are radioactive and that they emit radiation. In this context, radiation is more or less the generic term for "stuff emitted by various kinds of atomic processes that you probably don't want to come into contact with".
Unfortunately, the names of various types of radiation are incredibly confusing, dating from a time period where the physics of nuclear energy was poorly understood. When some new form of radiation was discovered physicists would tend to give it a name that just reflected that it was something new, hence "X-rays" (with the "X" indicating unknown) and alpha, beta, and gamma radiation, names (according to Wikipedia, based on the degree to which they penetrated matter). Now, of course, we understand the actual physics a lot better, but the old names persist. As a practical matter, you'll hear about the following:
| Name | What it actually is |
|---|---|
| Alpha | Helium nuclei (two protons and to neutrons) |
| Beta | Electrons |
| Gamma | High energy photons (i.e., light, but outside the visible range) |
| X-rays | High energy photons, but typically lower energy than Gamma |
| Neutrons | Neutrons |
These are all bad for you, but different levels of bad. None of them will turn you into The Hulk.
Fission #
Atoms can also undergo fission in which the nucleus splits into two smaller nuclei, some other particles such as neutrons, x-rays, etc. Most relevant to us are the following fission reactions, which we'll discuss shortly:
-
Uranium-235 can break up into (typically) krypton-92 and barium-141
-
Plutonium-239 can break up into (typically) zirconium-103 and xenon-134
I say "typically" because fission is kind of a non-deterministic process: the new nuclei need to have a mass that adds up to the original mass (minus whatever other particles were emitted) but there's some variation in which elements are produced. The following figure shows the distribution of fission products for some common fissile isotopes:
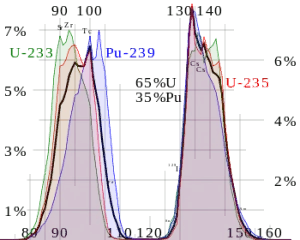
[From nuclear-power.com]
It's possible for atoms to spontaneously undergo fission (more on this later), but more commonly it's the result of external forces. Specifically, if a neutron impacts the nucleus of an atom it can attach itself to the nucleus, creating a new isotope that is one unit heavier. If this isotope is unstable (as is reasonably likely, because you're perturbing an isotope which is currently stable) it can undergo fission.
Chain Reactions #
Here's what we know so far:
- When an atom undergoes fission, it can emit neutrons
- When a neutron hits an atom, it can cause it to undergo fission
When you put these two facts together, you can have what's called a chain reaction in which one atom undergoes fission and produces enough neutrons to cause two atoms to undergo fission; in turn those atoms emit more neutrons, and we have an exponential growth process which results in the rapid release of very large amounts of energy, in other words, an atomic bomb.
The figure below shows the process, also helpfully showing that Uranium doesn't always decay into the same pieces.
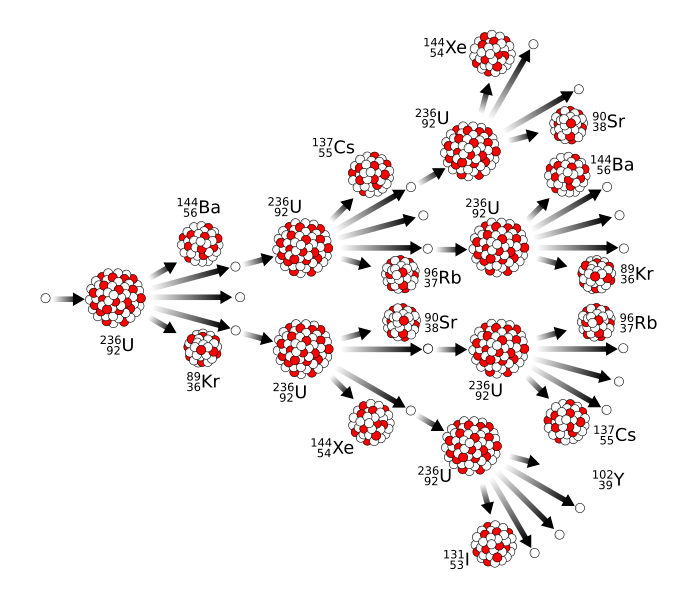
[Image by MikeRun from Wikimedia]
Note that it's not necessary for every neutron to impact a nucleus in order to get a chain reaction as long as on average the fission of one atom results in the fission of more than one other atom. Nuclear reactors work by modulating the number of neutrons that effectively impact other atoms, thus keeping a stable reaction rate rather than one that is explosively exponential. Describing how that works is outside the scope of this post, however.
Fusion #
It's also possible for two light atoms to come together to form one heavier atom, in a process called fusion. The most relevant case for us is that two hydrogen atoms (atomic number 1) can fuse to form one helium atom (atomic number 2). This is what happens in the sun, but can also be exploited to build a much bigger bomb than a pure fission bomb. More on this later.
Making an Atomic Bomb #
Once you have the insight from the chain reaction, it's a pretty straight shot to the idea of an atomic bomb, and physicist Leo Szilard famously invented it while waiting at a traffic light:
"In London, where Southampton Row passes Russell Square, across from the British Museum in Bloomsbury, Leo Szilard waited irritably one gray Depression morning for the stoplight to change. A trace of rain had fallen during the night; Tuesday, September 12, 1933, dawned cool, humid and dull. Drizzling rain would begin again in early afternoon. When Szilard told the story later he never mentioned his destination that morning. He may have had none; he often walked to think. In any case another destination intervened. The stoplight changed to green. Szilard stepped off the curb. As he crossed the street time cracked open before him and he saw a way to the future, death into the world and all our woes, the shape of things to come"...
[Quote from Richard Rhodes's "Making of the Atomic Bomb"]
It was almost 12 years from that moment when the first atomic bomb was tested at Alomogordo New Mexico. This test was the result of three years of work by over 100,000 people and an investment of over $23 billion in current dollars, in the form of the US Manhattan Project. This raises the question: if it's so straightforward, what took so long?
In order to get exponential growth you need to have on average more than one neutron emitted from the first fission event to create fission in some other atom. Otherwise, you get an exponential decay process where the chain reaction goes toward zero and nothing much happens. If you just have a small number of atoms, then the most likely thing is that a neutron will just be emitted outside your fissile material and not contribute to the chain reaction. You need a certain minimum amount of material in order to get the probability of subsequent fission high enough that you get exponential growth. This amount is called the critical mass and depends on the specific properties of the element you are using, and in particular (1) how many neutrons it emits when it undergoes fission and (2) how likely it is that when a given neutron hits an atom it will result in a new fission event. The critical mass also depends on the shape (geometry) of the fissile material, with a sphere being the ideal shape because it has the maximum volume to surface ratio, which minimizes the chance that the neutrons will just be uselessly expelled from the surface.
OK, so we just need to collect enough material and presto, we have a bomb. Unfortunately, it's not so simple:
- Getting enough of the right material is hard.
- As soon as you start to assemble the material into a critical mass, it starts reacting, and so if you do it wrong, the energy emission will cause it to explosively disassemble, which isn't fun if you're nearby, but produces a much smaller bang than you were looking for (a "fizzle").
Let's look at each of these in turn.
A Materials Problem #
First, we have the problem of the right material. It quickly became apparent that there was only one suitable natural element: uranium.
Uranium #
Recall that I said above that the uranium-235 nucleus (U-235) can easily undergo fission. Fortunately for us, but unfortunately for the purposes of making an atomic bomb, the 99% of the uranium in the world is not uranium-235 but rather uranium-238 (U-238), which does not readily undergo fission when bombarded by neutrons (instead, it tends to form U-239, which eventually decays but doesn't undergo fission, we'll want this information later). This presents a problem because it means that most of the neutrons emitted by U-235 fission don't lead to more fission events and you don't get exponential growth, hence no bomb. Or, more precisely, the critical mass of natural uranium was improbably large, between 10 and 44 tons (calculation by Rudolf Pierls, as cited by Rhodes). Not something you could drop from a plane.[4]
However, what people eventually realized was that if you had just U-235, or even mostly U-235, then it was possible to sustain a fission explosion with much less mass (the first uranium bomb, Little Boy, used 64kg of uranium). So, now the problem just becomes enriching the uranium so that you have a higher fraction of U-235 than in natural uranium (Little Boy used 80% U-235).[5] This is where things start to get hard.
Traditionally, there are two main ways to separate out a mixture of two substances:
-
Via chemical processes. For instance, this lab experiment describes how to separate out the components of a common headache medicine into acetylsalicylic acid (aspirin), salicylamide, and caffeine, by taking advantage of the fact that each component reacts differently with different reagents.
-
Via physical processes. For instance, given a mixture of alcohol and water (e.g., wine) you can increase the alcohol concentration in the mixture by heating it and collecting the vapor to produce brandy; this takes advantage of the fact that alcohol has a lower boiling point than water and therefore the vapor has more alcohol than the original liquid.
Unfortunately, because U-235 and U-238 are both isotopes of uranium, they behave chemically identically, so chemical processes are more or less impractical.[6] This leaves us with physical processes, but because the weight of the respective molecules differs by only 1.2%, the physical behavioral differences are very small as well, which makes any physical separation process very inefficient. Eventually, the physicists on the Manhattan Project settled on two main approaches.
Gaseous Diffusion #
In this approach, you create a gaseous form of uranium hexafluoride and allowed it to slowly diffuse across nickel barrier with very small perforations. Because the U-235 molecules are slightly lighter than the U-238 molecules, they move across the membrane slightly faster, with the result that if you stop partway the resulting mixture on the far side has slightly more U-235 than the starting mixture. Because this process is so inefficient, you need multiple stages in which the output of one stage is fed into another. To make matters worse, the uranium hexafluoride is fiendishly reactive and toxic, so very hard to work with. The result is difficult industrial chemistry on a giant scale.
Multiple Lines of Attack #
One thing that Rhodes does a great job of bringing out is the extent to which the Manhattan Project involved pursuing multiple lines of attack on the problem of building an atomic bomb, with the hope at least some of them would work. Some failed, of course, but at the end of the day, they had two entirely different routes that succeeded, with the result that the two bombs that were eventually dropped used totally different technologies: uranium "gun-type" devices and plutonium implosion devices. Similarly, they purused three independent technologies for uranium enrichment, of which two turn out to be really useful.
Electromagnetic Separation (mass spectrometry) #
The intuition here is that if you ionize the uranium atoms so that they have an electrical charge (I told you we'd come back to ions) you can then accelerate them with an electric field. If you then apply a transverse (perpendicular) magnetic field, then the ions will follow a curved trajectory, as shown below. Because the U-235 ions are slightly lighter they will follow a slightly tighter trajectory; you can then effectively set up a bucket and collect them. Of course, it will be a very small bucket because you are literally separating one atom at a time.
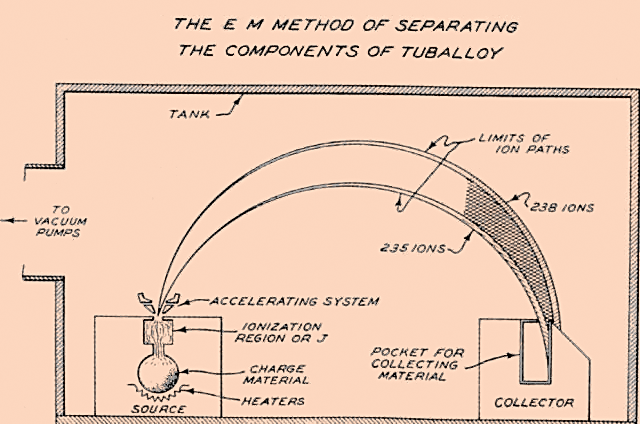
[The original diagram for electromagnetic separation.]
The scale of both of these processes was truly enormous. Rhodes again:
The United States was critically short of copper, the best common metal for winding the coils of electromagnets. For recoverable use, the Treasury offered to make silver bullion available in copper's stead. The Manhattan District put the offer to the test, Nichols negotiating the loan with Treasury Undersecretary Daniel Bell. "At one point in the negotiations," writes Groves, "Nichols ... said that they would need between five and ten thousand tons of silver. This led to the icy reply: 'Colonel, in the Treasury we do not speak of tons of silver; our unit is the Troy ounce.'"
The Manhattan project eventually ended up using both of these processes, gaseous diffusion first, and then electromagnetic separation.[7]
Even the more modern process involving high-speed centrifuges involves a fairly significant investment. However, there is a more easy way to get the fissile material you need to make an atomic bomb.
Plutonium #
Uranium is the only natural material suitable for making a bomb, but element 94 (plutonium) works fine as well well. Plutonium has two very convenient properties:
-
It's relatively easy to make with nuclear reactors because it's the result of U-238 reacting with a neutron (see above). So all you need is a nuclear reactor and some U-238 and you've got plutonium. In practice, reactors never run on pure U-235, so they always produce plutonium, even if it's treated as a waste product. Of course, you can design your reactor to optimize the production of plutonium.
-
Because plutonium isn't just an isotope of uranium it's relatively easy to chemically separate from the U-238 it was created in. I say relatively because both plutonium and uranium are highly toxic and the whole mess is intensely radioactive, but fundamentally it's just chemistry; no need for gaseous diffusion or mass spectrometers. Plutonium itself comes in several isotopes, but the isotope you get the most of, Pu-239, is the one you want for making bombs.
For these two reasons, modern atomic bombs generally use plutonium rather than uranium.[8]
Assembly #
Once you have your fissile material you need to assemble it into a critical mass. This is a challenging process, because, as noted above, once you start to bring the material together it starts reacting even before the critical mass is assembled. If you do it wrong, the energy emission will cause it to explosively disassemble, but with a much smaller bang than you were looking for (a "fizzle"). In order to make a bomb, you need to bring the material together very fast so that you get a lot of fission before the critical mass disassembles itself (i.e., explodes). Even so, you typically only get a fairly small proportion of the material reacting, but the reaction is so energetic that you still get a big explosion.
Gun-Type Devices #
Uranium bombs are comparatively simple to build, using what's called a "gun-type" assembly mechanism, as shown below:
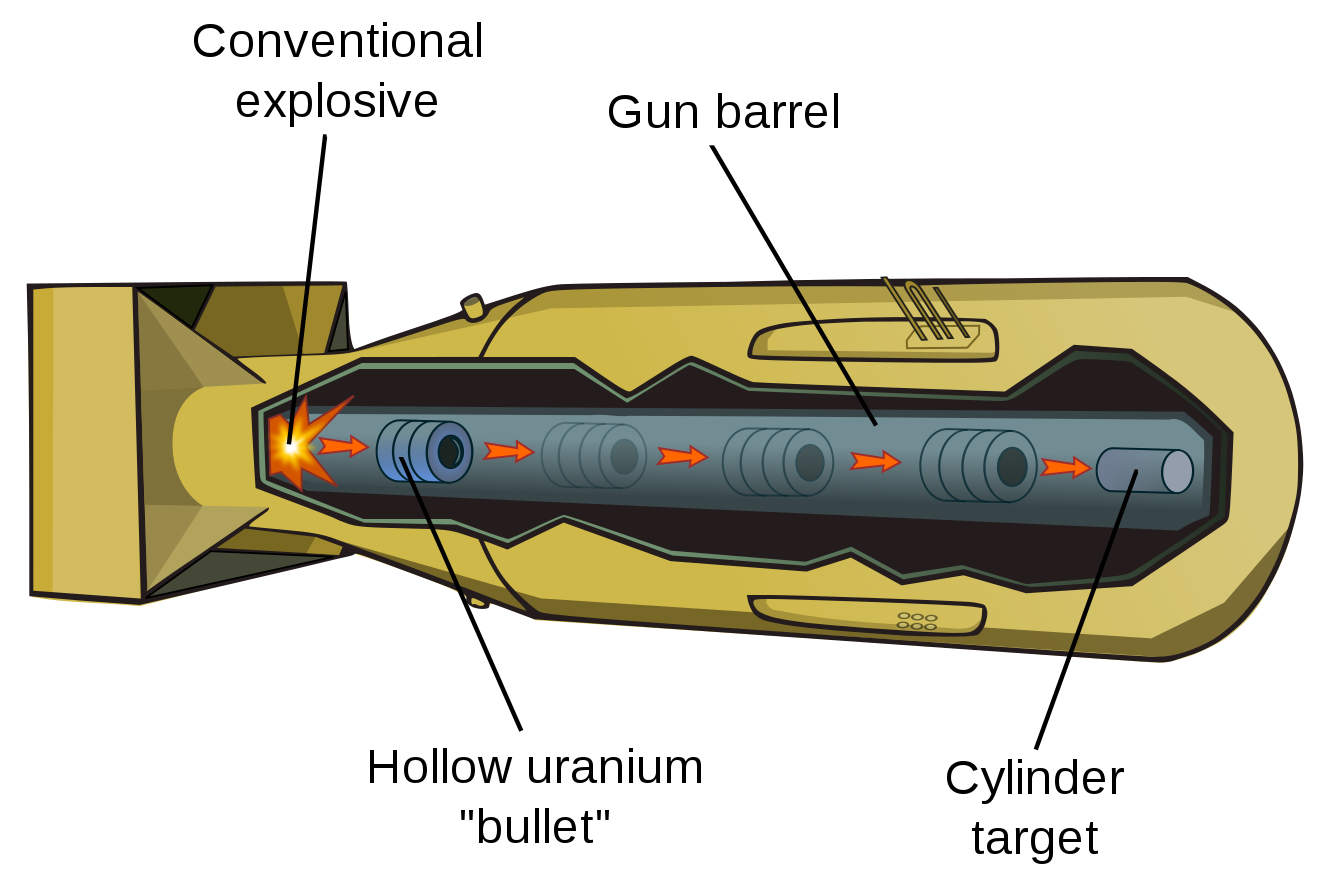
[Diagram by Dake, Papa Lima Whiskey, and Mfield from Wikipedia ]
This diagram shows a full weapon, but just focus on the gray area in the center that represents the "physics package", i.e., the atomic bomb itself, not the stuff needed to deliver it. Basically, a gun type bomb is what it sounds like: you have a hollow "bullet" made of uranium and you shoot it down a long barrel (originally literally made from a cannon) at a cylindrical "target" also made of uranium. When the cylinder contacts the target and surrounds it the result is a critical mass, resulting in an explosion. This all happens very quickly: you don't even need to have something to stop the bullet because the brief period when the target is passing through the bullet is enough. And of course, once the reaction starts, the whole thing will explosively dismantle itself anyway.
Implosion Devices #
You cannot, however, build a plutonium-based bomb using a gun-type mechanism. Reactor-manufactured plutonium is mostly Pu-239 but contains a small fraction of Pu-240, which has a relatively high rate of spontaneous fission. This rate is sufficiently high that as the bullet and cylinder start to assemble a critical mass, the reaction will start and the mass will prematurely disassemble, with the result that you get "fizzle" rather than a successful explosion.
Instead, plutonium bombs are built using what's called an implosion system, as shown in the diagram below:
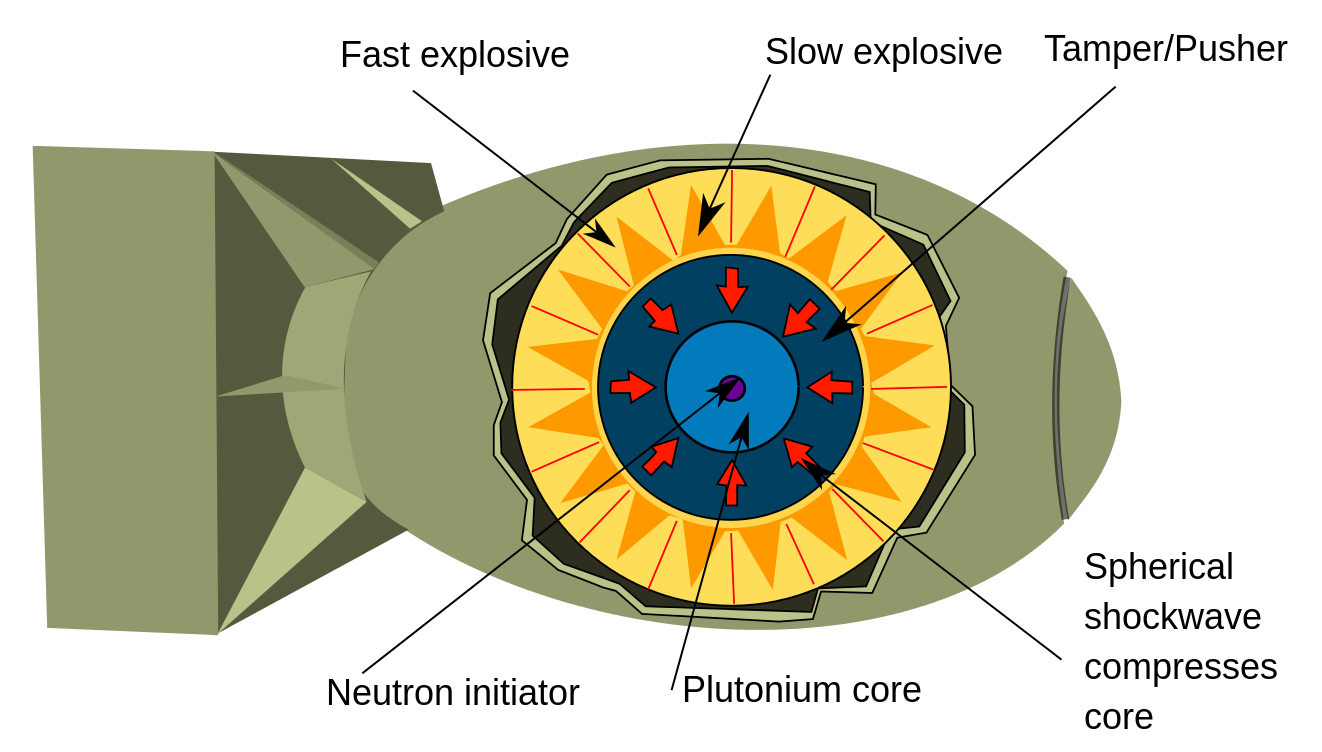
[Diagram by Ausis via Wikipedia]
In an implosion device you have a spherical core (sometimes hollow and sometimes solid)[9] called the "pit". It's surrounded by explosives which compress the pit in a spherically symmetrical pattern, thus forming a critical mass which holds together long enough to produce an explosion.
An implosion device is much less straightforward to build than a gun-type device, in large part because it's hard to get the explosives in the form of shaped charges to actually symmetrically compress the pit. As a comparison point, the world's first nuclear explosion was a test of an implosion-type bomb. The physicists at the Manhattan Project were so confident that the gun-type bomb would work that the first one ever detonated was the bomb dropped on Hiroshima, without any live testing at all.
Once you know how to do it, however, plutonium is much more convenient as a material to use for weapons because, as noted above, it's so much easier to obtain. Moreover, at this point it's fairly well understood how to build implosion devices, to the point where non-experts have famously designed plausible weapons without recourse to classified information. And of course, at this point 9 total countries have successfully built nuclear weapons (the US, Russia, the UK, France, China, India, Pakistan, North Korea, and Israel). In other words, the really hard part of building a nuclear weapon is getting the plutonium in the first place.
Thermonuclear Weapons #
Everything I've written so far is about fission type weapons, which are the original atomic bombs. However, modern weapons are frequently what's called "thermonuclear" devices which are based on both nuclear fission and nuclear fusion (aka "hydrogen bombs"). The details are of course complicated, but briefly, fusion takes place under conditions of very high heat and so you use a fission explosion (the "primary") to initiate the fusion reaction (the "secondary"). For reasons that are out of scope of this post, fusion bombs can be made much more powerful than fission-only bombs.
They're also substantially more complicated to design, because, like implosion devices, you have to ensure that they have time to fuse before they disassemble themselves. Wikipedia has a good primer on the design of thermonuclear devices. Richard Rhodes's Dark Sun contains a much more in-depth treatment of the history and design of thermonuclear weapons.
Disposal #
After 4500+ words, we're finally ready to address the question we started with, which is to say, how one disposes of unwanted nuclear weapons. As described in the aforementioned NYT article, the current practice in the US is mostly to disassemble them and to store the parts, making it possible to reassemble them later into similar weapons. The article leans kind of heavily on the fact that this is surprising (true!) but does eventually list three reasons why it might not be a good idea:
-
The parts themselves (principally the pits) are a safety hazard.
-
There are "security" issues, presumably that someone might steal the parts and make their own weapon.
-
That this doesn't really put them beyond use and so isn't a real reduction in the number of weapons because the US could readily make new weapons if it chose to.
There are two primary assets that we might need to concern ourselves:
- The plutonium pit itself
- The rest of the weapon
The situation with the rest of the weapon is simpler so let's look at that first.
The Rest of the Weapon #
The parts of the weapon other than the pit give you a head start on building a new weapon in two ways. First, if you just disassemble the weapon into pieces then it's (presumably) comparatively straightforward to reassemble them back into a functional weapon. You might also be able to reassemble them into a similar weapon though based on what I know, you would want it to be reasonably similar to the original. In either case, this is almost certainly easier than manufacturing all new parts and the necessary associated supply chain.

[A lightly modified version of Midjourney's output for "ikea instructions for assembling a nuclear weapon, diagram, black and white, detailed, realistic --v 4"]
Second, the parts embody the knowledge about how to build a new weapon. As noted above, while it's helpful to have this for building a fission device, at this point this is something that can be reproduced fairly readily. However, thermonuclear bombs are significantly more complicated to design and quite easy to get wrong, so it would definitely be helpful to have a reference design to start from. The fusion component also seems to involve some isotopes of hydrogen (tritium and deuterium), so it would be modestly helpful to have that but my understanding is that it's not that hard to get your hands on these isotopes. Deuterium in the form of "heavy water" (i.e., heavy hydrogen and oxygen) is readily available from chemical supply houses. So, while the article says "the nuclear warhead is the bullet-like cylinder at the back. It holds the plutonium pit and the hydrogen fuel, which gives the bomb its vast powers of destruction", my sense is that the hydrogen fuel part is pretty easy to obtain.
But of course none of this is very useful if you don't have the pit, which is necessary to start the whole thing off. It's also fairly straightforward to destroy these components, as they're fundamentally just hardware. Not so, for the pit.
The Pit #
The pit presents two problems. First, even without the rest of the components, the plutonium pits can be reused to make new weapons, either with a similar geometry to the current weapon, or melted down and formed into the pit of a new weapon with a new geometry. We know from experience that once state-level actors get access to enough plutonium to build a bomb they generally succeed. Of course, non-state-level actors might have a much harder time building a bomb from raw plutonium.
Second, it's extremely difficult to destroy plutonium effectively (some weapons are built out of highly enriched uranium and that can just be diluted in U-238 and used for reactors). Obviously, you can melt it down, but that just leaves you with a chunk of subcritical plutonium which someone can re-form into a new weapon. The plutonium is highly toxic, so you can't just grind it up and scatter it around without causing huge environmental impacts (watch Chernobyl if you want to get a sense of what I'm talking about here). You can't burn it because then you're going to have oxidized plutonium in the air, which you don't want people inhaling, and while you can of course use chemicals to dissolve it, vitrify it, etc. you're still left with an equivalent amount of plutonium, just bonded to some other stuff, and so it's just a matter of (potentially highly unpleasant) chemistry to get it back out again. In other words, it's precisely the properties of plutonium that make it attractive to build nuclear weapons out of that make it so hard to dispose of.
It's also very difficult to store because while an individual weapon may not be a critical mass, if you have tens or hundreds of weapons you have to worry about them getting close enough to worry about accidentally assembling a critical mass just from proximity, which, would of course, be bad.
Of course, this isn't news to policymakers. As the NYT article says:
The Clinton, Bush and Obama administrations all made plans — with costs in the billions of dollars — to get rid of excess plutonium stocks, which grew rapidly after the Cold War because of arms disassembly. But no strategy has so far succeeded.
The best available option appears to be seems to be to turn the plutonium into what's called "mixed-oxide fuel" (MOX) and then using it to fuel nuclear reactors. Unfortunately, this doesn't work super well for a number of logistical reasons, for instance that many reactors can only use MOX for some of their fuel; and of course we have an unbelievable amount of plutonium lying around, not just from existing nuclear weapons but also from the operations of normal nuclear reactors, which, as noted above, create plutonium. The FAS report I linked above is from 1993 and states that "There is almost 1000 MT of reactor Pu (R-Pu) in existence now, with the amount growing by about 100 MT per year." (disposal of plutonium waste is one of the big problems with nuclear reactors). So, the situation is really quite difficult even if we ignore disassembled weapons, which actually tend not to be that big (recall that the pit weighs on the order of a few kg).
Final Thoughts #
I don't want to spend too much time playing media critic here, but I don't feel like this article did that great a job of putting things in context. The implication of this article is that the US isn't really serious about disarmament and so it's storing all the nukes in pieces but not really destroying them in order to have ready access later, and that this creates all sorts of hazards. I'm sure that's true to some extent, but I think it's also necessary to realize that actually destroying them is a lot harder than it sounds and even if you were to do about the best we know how to do and totally destroy all of the hardware other than the pits, you'd still be left with a large amount of fantastically dangerous stuff which has to be guarded for the next 100,000 years or so. The critique that this material isn't being guarded does seem like a reasonable one, but it seems like guarding it better is the solution that we're left with.
In reality this whole orbiting thing is kind of nonsense because actually they occupy this probability space of locations, but we don't need to get into quantum mechanics here and for our purposes we can just live with a classical-type picture. ↩︎
Technically, this is called the atomic mass. Protons and neutrons have approximately the same mass, but electrons are much lighter, so the mass of the atom is basically the mass of the protons and neutrons. ↩︎
This "m" isn't an error. I didn't know about this, but apparently this is actually a higher energy state of protractinium-234, which decays more quickly. Thanks, Wikipedia! ↩︎
Famously, the Oklo Mine had a self-sustaining reaction in natural uranium, though with the help of water as a "moderator" (out of scope again, I'm afraid). ↩︎
The uranium with lower than normal U-235 is known as depleted uranium and is used in various military applications because it is very dense. ↩︎
There actually is now a chemical process called Chemex that takes advantage of some slight differences in chemical properties due to the change in atomic mass. ↩︎
There were actually three separate processes, with thermal diffusion being used to make slightly enriched uranium which was then enriched much more with gaseous diffusion. Thermal diffusion isn't very efficient and was eventually abandoned. ↩︎
Note that you still need the ability to enrich uranium to reactor grade levels so that you can run the reactor to make the plutonium. ↩︎
The original pits were hollow, but as I understand it more modern designs just use a solid pit and rely on the explosives to compress the plutonium enough to make a subcritical mass critical. ↩︎